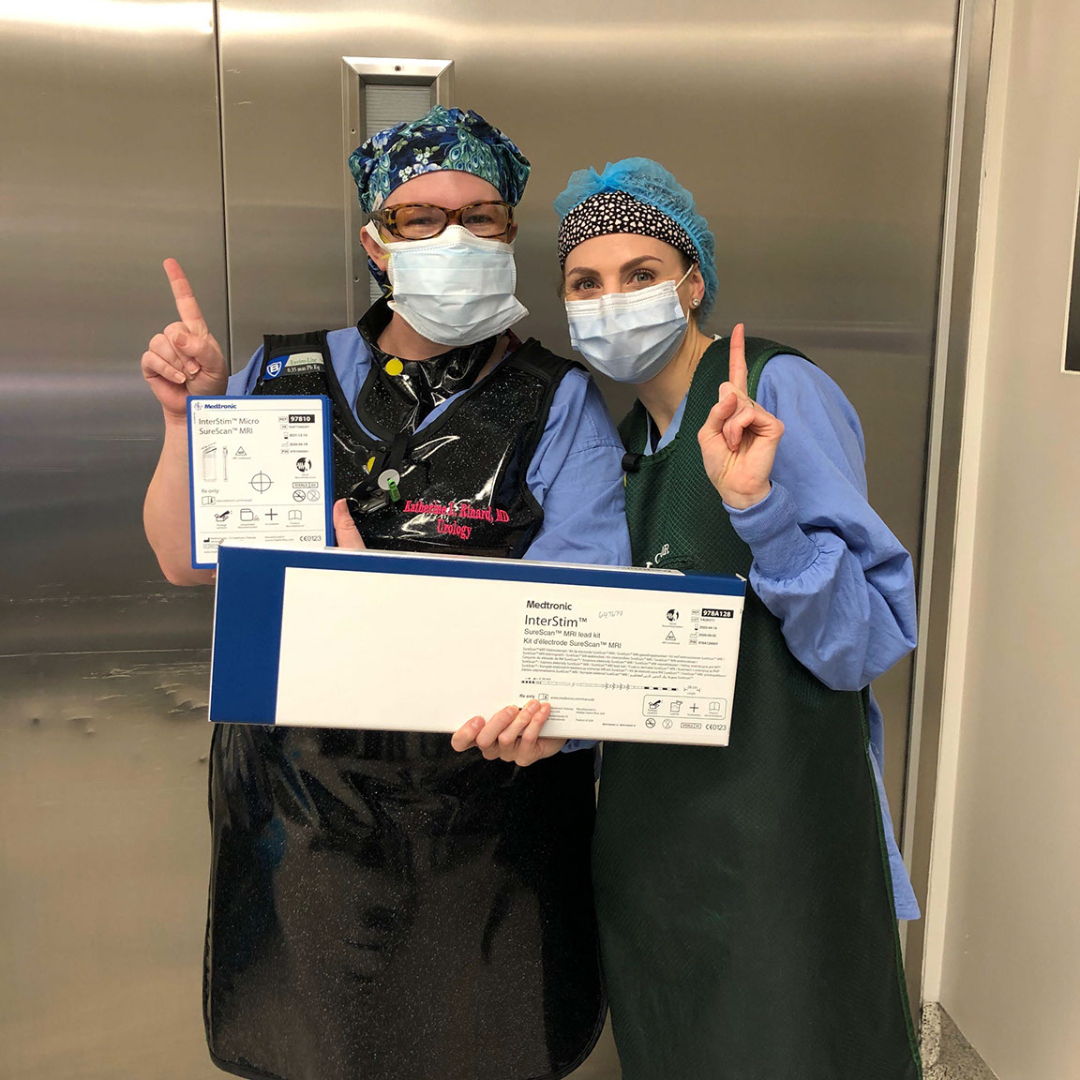
First Hendrick patient undergoes innovative surgery for bladder and bowel control issues

Hendrick Health is now offering an innovative solution to bladder and bowel control conditions in patients. The InterStim Micro neurostimulator and InterStim SureScan MRI leads were approved by the U.S. Food and Drug Administration (FDA) in August, for the treatment of patients with bladder and bowel control conditions, including overactive bladder (OAB), fecal incontinence (FI) and non-obstructive urinary retention. Dr. Katherine Rinard, urologist at Hendrick, performed the hospital’s first procedure on Tuesday, Sept. 22.
”Sacral neuromodulation helps patients with these often debilitating issues when other treatments have failed,” said Rinard. “While the ability to control your bladder and bowel is usually not dangerous, these issues can severely affect quality of life. Most of us take for granted going to the store, visiting friends or even sleeping through the night. This device acts as a ‘pacemaker’ to help the bladder and brain communicate more normally.”
The InterStim Micro, currently the smallest rechargeable device to deliver sacral neuromodulation (SNM) therapy, sends electrical impulses to the nerves located in the lower back to improve control. Oral medications only target the muscular component of bladder control, but SNM offers symptom control through direct modulation of the nerve activity.
“We are also very excited that the device is MRI compatible,” said Rinard. “Many of my patients have coexisting medical problems, like multiple sclerosis or back injuries that require specialized imaging. Until now, these patients have been excluded from undergoing sacral neuromodulation surgery.”